Group 18 Noble Gases Are Relatively Inert Because
Their outermost energy level is missing one electron. Compared to other element groups.

Noble Gases Are Mostly Inert Assign Reasons
The noble gases Group 18 are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact that their filled valence shells octets make them extremely nonreactive.

. Carbon and other nonmetals are found in which area of the periodic table. Elements within the same group in the periodic table have similar properties because they have the same number of. The noble gases were characterized relatively late compared to other element groups.
Consistent with periodic trends in atomic properties these elements have high ionization energies that decrease smoothly down the group. Because this configuration is extremely stable as well as symmetrical the noble gases are very unreactive. They were once called inert gases because they were thought to be completely inertunable to form compounds.
They are all odorless colorless monatomic gases with very low chemical reactivity. The noble gases are in Group 18 8A. Properties of the Noble Gases.
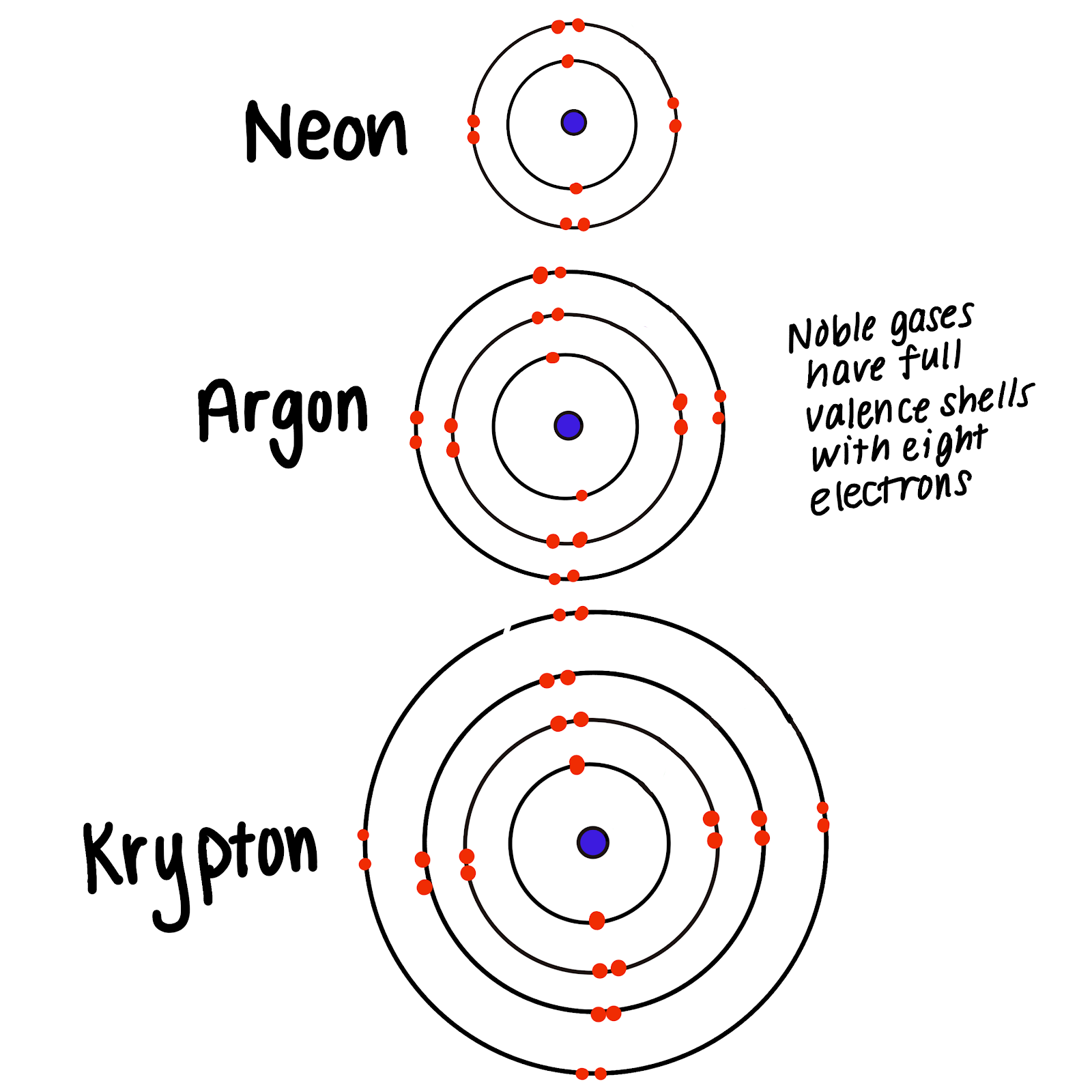
Noble gases are relatively inert and nonreactive due to their full outer shell of electrons. The noble gases are group 18 elements of the periodic table. Transition metals such as copper or tungsten form compounds by.
Group 18 noble gases are relatively inert because _____ They are reactive found as compounds soft shiny have similar melting and boiling points and have similar densities. Atoms of these elements have completely filled valence electron shells making them relatively inert colourless odourless monatomic gases at room temperature and pressure. Properties of Nobel Gases.
Their s and p orbitals are filled. Group 18 noble gases are inert because. From their electron affinities the data in Table 223.
The noble gases were characterized relatively late compared to other element groups. 1 indicate that the noble gases are. They readily form positive ions.
The six noble gases that occur naturally are helium He Neon Ne Argon Ar Krypton Kr Xenon Xe and Radon Rn. A groups elements react similarly because of their _____. They are helium neon argon krypton xenon and radon.
Group 18 noble gases are relatively inert because. Group 18 of the periodic table contains the noble gases. Which element is a semiconductor.
Their outermost energy level is full. Elements in a family often have similar. They can have either a positive or negative charge.
In his version Mendeleev based his arrangement of the elements on an elements. The elements of group 18 all have closed-shell valence electron configurations either ns 2 np 6 or 1s 2 for He. The noble gases Group 18 are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact that their filled valence shells octets make them extremely nonreactive.
Group 18 noble gases are relatively inert because _____ Their s and p orbitals are filled. On the right side. These are the elements in the 18th column of the periodic table at the far right.
One of the important ideas about Mendeleevs periodic table was that he predicted new _____. These gases all have similar properties under standard conditions. Group 18 noble gases is located at the far right of the Periodic Table of elements and is simply referred to as inert gases because they are extremely non - reactive due to their filled valence shells octets.
The noble gases Group 18 are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact that their filled valence shells octets make them extremely nonreactive. The outer level is full. They are all nonmetals and are found in their standard state as monatomic gases.
Group 18 noble gases are relatively inert because a. The noble gases are a group of chemical elements that make up Group 18 on the periodic table. Losing electrons to form positive ions.
Group 18 is the noble gases that were once known as inert gases because they are relatively unreactive.

Please Select A Team 1 Females 2 Those That Aren T Female Ppt Download

Comments
Post a Comment